INTRODUCTION
In the recent years we have witnessed a distinctly higher interest in the interplay between the function of the intestines, intestinal microflora, dysfunction of the continuity of intestinal barrier and the function of the Central Nervous System (CNS). These interrelations take place in the so-called gut-brain axis that is the brain-gut-microbiota axis. Frequent comorbidity of dysfunctions within the intestines, such as irritable bowel syndrome (IBS) or inflammatory bowel diseases (colitis ulcerosa or Crohn's disease) (Qin et al., 2010) and mental disorders, mostly depression and anxiety, points us in the direction of significant relations and interactions within the gut-brain axis. Relationships of this kind also occur in schizophrenia and autism. In fact a hypothesis of schizophrenia having its source in the digestive system appeared as early as at the beginning of the 20th century. A post-mortem study of 82 patients suffering from schizophrenia, conducted by Buscaino, revealed numerous inflammations in their gastrointestinal tracts, such as gastritis (50%), small intestine inflammation (88%) and colitis (92%) (Hemmings and Hemmings, 1978). These changes were partly similar to the histological changes characteristic of Coeliac disease. Also Asperger noted a relationship between Coeliac disease and psychotic disorders (Apserger 1961). Moreover, studies of the impact of gluten-free diet on people suffering from schizophrenia have demonstrated its therapeutic effect in some patients (Singh and Kay 1976). Professor Henri Baruk, the French authority in the field of psychiatry, emphasised the relevance of the gastrointestinal tract, intestinal toxins and infectious agents in the context of catatonia and schizophrenia, based on his 50-year experience and extensive research into schizophrenia and catatonia which he conducted at the Sorbonne. (Baruk 1953; Baruk and Camus 1958; Baruk and Fabiani 1962; Hemmings and Hemmings 1978). The gastrointestinal tract seems to play the key role in the pathogenesis of autism, which was confirmed by Wakefiled at al. (1998, 2000) demonstrating various inflammatory changes within the gastrointestinal tract of children suffering from autism. Dysfunctions in the gut-brain axis are also linked with the abdominal pain syndrome and eating disorders (Mayer 2011). In the context of this research, modulating of the gut-brain axis can become a starting point for development of new treatment strategies for many illnesses, from mood and anxiety to gastrointestinal tract disorders. It is also possible that some appropriate dietary interventions, such as reducing gluten-containing products, may prove helpful in the treatment of mental diseases.
Intestinal microbiota that is intestinal bacterial flora, is one of the key elements of the gut-brain axis and therefore it deserves a special attention. In the recent years a lot of studies confirmed its impact on mood and behaviour, mainly through the regulation of response to stress and reduction of depressive symptoms and anxiety (Rhee et al., 2009). It has a direct impact on the modulation of the concentration of pro-inflammatory and anti-inflammatory cytokines; it affects the tryptophan metabolism in the kynurenine pathway, in this way modulating serotonin content, and it also affects the functioning of the hypothalamic-pituitary-adrenal axis (HPA axis), produces numerous neurotransmitters and improves the "tightness" of the intestinal barrier - the largest (400-600 m2) surface, through which our organisms are in contact with the external world.
There is a lot of evidence for the activation of inflammation in depression, which manifests itself, among others, by an increased concentration of pro-inflammatory cytokines IL-1β, IL-5, IL-6 and tumor necrosis factor TNF-α, by an increase in the plasma concentration of positive acute phase proteins, such as haptoglobin, and a decrease in negative acute phase proteins, such as the albumin or transferrin (Maes 1995; Song et al., 1994; Myint et al., 2005). Frequent co-occurrence of depression with many inflammatory diseases such as rheumatic conditions, inflammatory bowel diseases (IBD), COPD, HIV, multiple sclerosis, coronary artery disease, Alzheimer's disease and others indicates the psychoneuroimmunological foundations of many mental disorders. The influence of psychological stress on the activation of inflammatory parameters, including increase in the concentration of pro-inflammatory cytokines, increased permeability of the intestinal barrier (Kiliaan et al., 1998; Demande et al., 2006) and the development of the so-called called leaky gut syndrome, as well as its impact on the composition and function of the microbiota (Rhee et al., 2009; Freestone et al.; 2002 Kiliaan et al.,1998; Bailey et al., 2004) also sheds additional light on the issue of the importance of many of the "residents" of the gut for the operations of the CNS and the brain-gut-microbiota axis.
"The Bulgarian Bacillus"
Probiotics (gr. pro bios = for life) are, according to the definition of the World Health Organisation (WHO) and the UN Food and Agriculture Organisation (FAO) "live microorganism, which when taken in a right amount, transfer their health promoting qualities to the new host". We owe our first observations of the therapeutic impact of bacteria on human health to the grandfather of probiotics, Ilia Mechnikov, who was the first scientist to study the relationship between the excellent health and long life of Bulgarian rural populations and the fact that they regularly drink sour milk, including milk acid bacteria, which he called the "Bulgarian Bacillus". The first reports on the attempts to include probiotics in the therapy of mental disorders were made at the beginning of the 20th century. In 1910, dr George Porter Phillips described a beneficial impact of milk acid bacteria on patients suffering from depression (Phillips 1910). In 1923, a group of researchers was behind the recommendation of the Acidofilus bacteria as the "agent causing physical improvement in the treatment of psychosis" (Julianne et al., 1923).
"A forgotten organ" – unusual composition, unusual functions
Microbiota is often referred as the "forgotten organ" because of its huge amount and the complexity of functions it fulfils. On average, the weight of microbiota present in the gastrointestinal tract of an adult is 1-2kg. Our intestines are, in fact, inhabited by approximately 1013–1014 microorganisms, which is 10 times the number of cells in the human body. These microorganisms include 150 times more genes than the human genome (Qin et al., 2010). This is an environment dominated by anaerobic bacteria and by two types of bacteria: Bacterioides and Firmicutes (Xu et al., 2007). Microbiota plays a number of functions within the gastrointestinal tract, such as immunomodulatory roles (O'Toole and Cooney 2008); it influences changes in the cytokines' levels, also by an interaction with GALT (gut associated lymphoid tissue), i.e. a lymphoid tissue of the gastrointestinal tract - the largest lymphatic organ in the human body, in which 70-80% of the cells of the immune system are formed. Intestinal bacteria also plays a protective role, by the receptor competing on the surface of the intestinal epithelium with pathogenic bacteria, competing with them for nutrients. It produces many antimicrobial agents, such as bacteriocins (Cryan et al., 2012), plays a structural role enhancing the tightness of the intestinal barrier, also by affecting the expression of some of the structural proteins constituting the so-called tight junctions between enterocytes. It also induces the synthesis of protective immunoglobulin A.
Microbiota is also involved in a lot of metabolic processes, including affecting the proliferation and differentiation of the as it supplies the epithelium layer with energy source, such as butyrate, short chain fatty acids (SCFA). It is engaged in the transformation of steroids intestinal epithelium and fatty acids, fermentation of dietary fibre, ion absorption, and it also synthesizes several vitamins B, vitamin K, and inhibits the growth of carcinogen-synthesizing bacteria, and is even able to metabolize certain carcinogens in food (Cryan et al., 2012).
The brain-gut-microbiota axis
It has been known for a long time that the brain regulates intestinal function, but it has been only recently recognized that the reverse is also true: intestinal processes and intestinal microbiota have an influence on the CNS. Guts, along with intestinal flora, and the brain are closely connected through the gut-brain axis (more specifically, the gut-brain-microbiota axis), which is a bidirectional communication route involving neural, endocrine and immunological mechanisms. Neural mechanisms include the enteric nervous system (ENS), with many neurotransmitters and neuromodulators, including serotonin, acetylcholine and corticotropin and corticoliberin (corticotropin releasing factor – CRF). CRF deserves a special mention because of its share in increasing the permeability of intestinal barrier under stress (Gareau et al., 2007, Collins et al., 2012). The next item on the gut-brain axis, i.e. the autonomic nervous system (ANS), consists of sympathetic and parasympathetic branches. The vagus nerve (n.X), which is a parasympathetic branch of the ANS deserves particular attention in this context. It provides a vital line of communication between intestinal microbiota and the CNS. Numerous studies have shown that pro-inflammatory cytokines may exert a direct effect on the CNS through the activation of afferent nerve fibres that transmit stimuli to the corresponding regions of the brain, such as the solitary nucleus (Irwin and Miller 2007), whereas efferent innervation can mediate the anti-inflammatory response, acting on the alpha7-nicotinic receptors, also in many cells of the immune system, reducing the secretion of inflammatory cytokines (Pavlov and Tracey 2004). Nemeroff et al. (2006) demonstrated that stimulation of the vagus nerve had an antidepressant effect and caused normalization of the HPA parameters in patients treated for recurrent depression (O'Keane et al 2005).
The endocrine factors regulating the gut-brain axis include cortisol, whose release under stress is regulated by the HPA axis, and which may affect immune cells by modulating the secretion of cytokines, the composition and functions of microbiota. Furthermore, intestinal bacteria have the ability to manufacture a number of neurohormones, such as serotonin, melatonin, GABA (γ-Aminobutyric acid), catecholamines, histamine, acetylcholine; they also produce short chain fatty acids (SCFA). All of these substances are likely to participate in communication within the intestinal bacterial flora; they may also play a peripheral and systemic role and affect brain function and behaviour (Iyer et al., 2004). A good example are the changes in the SCFA content in the faeces of children with autism (Wang et al 2012). In animal studies, propanoic acid (a short chain fatty acid overproduced by the intestinal bacteria in autistic children) fed to the rat CNS resulted in the occurrence of autistic behaviour and aggression (Thomas et al 2012). Moreover, there are reports of temporary success of the vancomecin treatment of aggression in people with autism.
Microbiota and probiotics may exert a direct effect on the immune system (Forsythe et al., 2010, Duerkop et al., 2009). Numerous studies have shown that intestinal bacteria can lower the levels of proinflammatory cytokines TNF-alpha, IFN-gamma, IL-6, and modulate the concentration of anti-inflammatory cytokines, such as IL-10 (Desbonnet et al., 2009).
Proinflammatory cytokines IL-1, IL-6, TNF-α, IFN-γ play key roles in the activation of the HPA axis. IL-1b, IL-6 and TNF-α increase the permeability of intestinal barrier with the subsequent, further activation of inflammation; in addition TNF-α, IFN-α and IFN-γ activate indoleamine 2, 3-dioxygenase (IDO) enzyme, which causes tryptophan transfer from the serotonin-producing tract to metabolism in the kynurenine pathway, reducing its concentration and increasing the concentration of tryptophan metabolites with neurotoxic and neuroexciting effects on the CNS, such as quinolinic acid and 3-hydroxykynurenine (Wichers and Maes 2004, Maes et al., 2011, Myint et al., 2007). In addition, cytokines can have a direct impact on the CNS through various mechanisms, such passing through the regions of the blood-brain barrier permeable for certain cytokines, by specific transporters, or by activation of afferent nerve fibres, such as the vagus nerve (Irwin and Miller 2007). It is therefore possible that the antidepressant and anxiolytic effect of intestinal bacteria may occur also by influencing the above mentioned elements.
The influence of stress on the permeability of intestinal barrier
The key role in the bidirectional connection between the CNS and the function of the intestines is played by an influence of psychological stress on increasing intestinal permeability, with subsequent translocation of bacteria from the intestinal lumen (Demande et al 2006) and the activation of inflammatory response by bacterial lipopolysaccharide (LPS), with an increase in pro-inflammatory cytokines. There is also an increasing number of reports on a potential role of increased permeability of intestinal barrier for food allergens and food allergy type III dependent on IgG (immunoglobulin G), in the context of depressive disorders (Rudzki et al 2012). Animal studies demonstrated that chronic psychological stress caused a 30-fold increase in the uptake of the Escherichia coli bacteria by GALT, with subsequent initiation of the pro-inflammatory immune response within the intestines (Velin et al 2004). Stress caused an increase in the sensitivity of the intestinal lymphoid tissue to food allergens (Yang et al., 2006) and initiated increased permeability of intestinal barrier for intestinal bacteria, and their increased uptake in the mesenteric lymph nodes, which was prevented by the supply of a mixture of probiotic Lactobacillus rhamnosus and L. Helvetius (Zarei et al., 2006). The role of increased permeability of intestinal barrier in the context of pathogenesis of depression was first confirmed by Maes et al. (2008), who demonstrated considerably increased levels of IgM and IgA immunoglobulins against the lipopolysaccharides of Gram-negative enterobacteria, regularly present in intestinal lumen. An increased secretion of CRF by locally acting immune cells, sympathetic neurons and enterochromaffin intestinal cells plays the key role in the mechanism of impaired, stress-induced permeability of intestinal barrier (Gareau et al, 2007). This is also one of the crucial mechanisms by which psychological stress can cause an increased translocation of bacteria from intestinal lumen, leading to the activation of inflammation. Besides it has been shown that psychological stress causes an increased concentration of some pro-inflammatory cytokines, such as IFN-gamma, which adversely affect the continuity of intestinal barrier (Kiliaan et al 1998). Also stress reduces the mRNA ZO-2 and occludin TJ expression, which are proteins comprising the tight junctions between enterocytes, which results in further deterioration of intestinal barrier function (Kiliaan et al 1998).
The influence of intestinal microbiota on the CNS
Sudo et al., (2004) were the first to demonstrate the impact of intestinal bacterial flora on correct development and functioning of the HPA axis. They based their research on mouse studies, using GF (germ free) mice, bred in entirely sterile conditions, deprived of any intestinal bacterial flora, and SPF (specific pathogen free) mice, stripped of particular pathogens. Under stress, an excessive rise in the concentrations of corticotropin (ACTH) and corticosterone was observed in the GF but not in the SPF mice. The stress response in the GF mice was then partially reversed by their colonization with faecal content derived from the SPF mice, and completely reversed by mono-association with the Bifidobacterium infantis bacteria, the dominant bacteria in the intestines of infants and a frequently used probiotic (Bailey and Coe 1999). One of the most surprising discoveries was that the reversal of the excessive activation of the HPA axis by Bifidobacterium infantis had its consequences in adulthood, but only in case of mice colonized with bacteria prior to 6 weeks of age. Colonization in the 14th week turned out to be entirely ineffective which indicates the existence of certain "window of susceptibility" to the effects of interaction between the bacteria and the host. Further research by Sudo (2006) demonstrated reduced content of brain-derived neurotrophic factor (BDNF), noraderenaline and 5-HT in the cortex and hippocampus in the GF mice as compared to the SPF mice. Desbonnet et al. (2009) studied a potential antidepressant activity of the Bifidobacterium infantis bacteria in rats by submitting them to the forced swim test (FST). The authors reported a significant decrease in the concentrations of pro-inflammatory cytokines IFN-gamma, TNF-alpha and IL-6, and reduced concentration of IL-10 in the group receiving the probiotic, compared to the control group. Besides, the probiotic increased the content of tryptophan and its metabolite, kynurenic acid, exhibiting neuroprotective activity through its antagonism on NMDA receptors. Another key finding in this study was that the group which received the probiotic, displayed a reduced content of 5-HIAA (5-hydroxyindoleacetic acid), a serotonin transformation product, in the frontal cortex, along with a reduced content of DOPAC (3,4- dihydroxyphenylacetic acid) – a dopamine metabolite in the cortical amygdala. In another study aimed at the assessment of potential antidepressant activity of bacteria Bifidobacterium infantis, young rats were subjected to stress by separating them from their mother (Desbonnet et al 2010). A probiotic caused, to a lesser extent than citalopram, a clear normalization of immune response, lowering the concentration of IL-6, reversed behavioural deficits and restored the initial concentration of noradrenaline in the brain stem. γ-Aminobutyric acid, as the main inhibitory neurotransmitter in the CNS, plays an essential role in physiological processes. Changes in the central expression of the GABA receptors are involved in the pathogenesis of anxiety disorders and depression, which are often comorbid with functional bowel disorders. Javier et al. (2011) demonstrated in the animal studies the influence of the Lactobacillus rhamnosus (JB-1) bacteria on the expression of the GABA receptors in the CNS and that the modulation involves the vagus nerve. Moreover the probiotic caused the reduction of corticosterone content and limited behaviours associated with depression and anxiety. Neurochemical and behavioural influences of probiotics were absent in mice subjected to vagotomy, indicating that the vagus nerve is a key element of communication between intestinal bacteria and the CNS. Girard et al (2009) showed that a mixture of probiotic Lactobacillus helveticus and Bifidobacterium longum prevented apoptosis of the limbic system and depressive behaviour in rats after a myocardial infarction. Interestingly, previous studies had shown that apoptosis may also be blocked by antidepressants (Wann et al 2009) or anti-inflammatory drugs (Kaloustian et al., 2007). Studies involving patients have also produced optimistic conclusions. Messaoudi et al. (2011) demonstrated that a combination of L. helveticus r0052 and B. longum R0175 showed anxiolytic activity, significantly reducing anxiety-related behaviour in rats, and easing the level of psychological distress in volunteers measured by the severity of somatization coefficient, anxiety, depression and aggression-hostility factor. The cortisol content was also reduced. Another research (Rao et al., 2009) which involved patients with chronic fatigue syndrome (CSF) who received Lactobacillus Casei Shitora (LcS) on a daily basis for 2 months produced a significant increase of bacteria Lactobacillus and Bifidobacteria in the patients' faeces and decrease of anxiety symptoms as compared to the group which received placebo. Other studies undertaken by Cazzola et al., (2010), Wagar et al. (2009) indicated that L. helveticus R0052 reduced the levels of IL-1b and IL-6, and statistically insignificantly of TNF-alpha, whereas Bifidobacterium longum R0175 reduced the levels of IL-8 and TNF alpha.
Ill. 1 Potential role of increased permeability of intestinal barrier (leaky gut syndrome) and activation of inflammatory immunological response in the pathogenesis of depression as well
as the mechanisms of the effects of microbiota on the CNS (partly adopted from Rudzki et al., 2012)
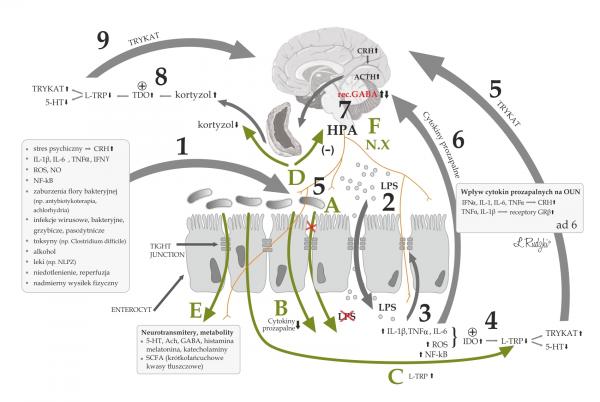
1) Factors which increase the permeability of the intestinal barrier and the weakening of tight junctions (TJ); 2) Increased translocation of bacteria in the intestinal lumen, following the weakened continuity of the intestinal barrier and activation of immune system by bacterial lipopolysaccharide (LPS), activation of inflammation with increased concentrations of proinflammatory cytokines, intensified oxidative stress, activation of the NF-kB - nuclear transcription factor; 3) further weakening of intestinal barrier and damage to the TJ under the influence of proinflammatory cytokines, oxidative and nitro-oxidative stress (ROS, NO) and NFkB activation; 4) Activation of 2,3-dioxygenase indoleamine (IDO) by pro-inflammatory cytokines and oxidative stress, with subsequent reduction of the content of tryptophan and serotonin (5-HT) and increased concentrations of tryptophan catabolites (TRYCAT); 5) harmful effect of TRYCAT on the CNS; 6) the influence of proinflammatory cytokines on the CNS through increasing the content of CRH and activation of the HPA axis, the development of glucocorticoid resistance by increasing the expression of an inactive form of the glucocorticoid receptor GRβ in relation to active GRα; 7) activation of the HPA axis and the subsequent increase in the cortisol content; 8) Activation of 2,3 dioxygenase tryptophan (TDO) by cortisol, with subsequent decrease in concentrations of tryptophan and 5-HT and the increase in the concentrations of tryptophan catabolites (TRYCAT); 9) harmful influence of TRYCAT on the CNS (partly adapted from Rudzki et al., 2012).
Mechanisms of the effects of microbiota on the CNS
A) Microbiota's prevention of an increased permeability of the intestinal barrier by the bacterial LPS and inhibiting the activation of inflammation
B) Reducing the levels of proinflammatory cytokines
C) Increasing tryptophan content (a serotonin precursor) and preventing the formation of TRYCAT
D) Inhibiting the activity of the HPA axis and reducing cortisol content
E) Producing a number of neurotransmitters and neurometabolites operating peripherally and systemically
F) Influence of microbiota on the CNS with afferent innervation (e.g., nerve X) and subsequent changes in the amount of the GABA receptors in the CNS.
The influence of stress on the composition and functions of microbiota
The activities within the gut-brain axis are bidirectional. In addition to the impact of microbiota on the function of the CNS, many studies have shown that psychological stress affects the composition and functions of microbiota. Environmental stressors have a negative impact on the microflora, and may increase the amount of aerobic bacteria and reduce the amount of lactobacillia in laboratory animals (Suzuki et al., 1983 Timoveyev et al., 2002). In humans, sudden emotional stress causes long-term reduction in lactobacillia and bifidobacteria (Moore et al., 1978, Holdeman et al., 1976). In addition, bifidobacteria seem to be very sensitive to stress and overexertion (Lizko et al 1987).
It has been also demonstrated that the separation from the mother causes a change in the composition of the microbiota in the neonatal rhesus monkeys (Bailey et al 1999). In addition, antenatal stressors may affect the composition of microbiota in the offspring of rhesus monkeys, reducing the total amount of bifidobacteria and lactobacillia. These changes may also result in increased susceptibility to infection and they indicate a mechanism of the influence of the mother's mental state during the pregnancy on the composition and functions of the infant's microbiota and overall health (Bailey et al., 2004). Another research showed that stress hormones had an impact on the increase of non-pathogenic isolated Escherichii coli and pathogenic strains of E. coli 0157:H7, through interaction with the host's catecholamines, such as adrenaline and noradrenaline (Freestone, et al., 2002).
CONCLUSIONS
New therapeutic prospects seem to emerge owing to the dynamically developing research of the gut-brain axis and interactions between the intestinal bacterial flora and the functioning of the CNS, as well as their role in the pathogenesis of mental disorders, mostly of depression and anxiety. This leads to a broader, more holistic view of the mentally ill and mental illness, including depression and anxiety disorders, as psychoneuroimmunological disorders in which the pathophysiology of inflammatory processes are often key elements. In this context, it seems justified to look at the issue from the following perspective: the intestines might serve as the "gate" and stress might be a "lock-pick" for the activation of the immune system, increased permeability of intestinal barrier, impact of proinflammatory cytokines on the metabolism of tryptophan and its metabolites, and on a number of mechanisms for the impact of proinflammatory cytokines on the CNS. Intestinal bacteria, due to the influence of the above mechanisms and individual elements of the gut-brain axis, can provide a valuable assistance in the treatment of mental disorders, as well as contribute to the prevention of the effects of stress, including preventing an increased permeability of intestinal barrier. Further studies of intestinal microbiota, and the effect of probiotics on the functions of the CNS and their therapeutic mechanisms in the context of psychiatric disorders can become a milestone in the improvement of the effectiveness of the treatment of mental illness, and further exploration of these mechanisms.
REFERENCES
- Asperger H. Die Psychopathologie des coeliakakranken kindes. Ann Paediatr 1961; 197: 146–151.
- Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol 1999; 35: 146–155.
- Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatr Gastroenterol Nutr 2004; 38, 414–421.
- Baruk H. Digestive and hepatointestinal etiology of the various mental diseases. Schweiz Med Wochenschr. 1953 Sep 19; 83(38 Suppl.): 1517–1518.
- Baruk H, Camus L. Biliary & hepatic poisons in pathogenesis of schizophrenia; experimental study. Confinia neurologica 1958, 18 (2–4), 254–63.
- Baruk H, Fabiani P. Study of blood ammonia in periodic psychosis and in the epileptic state. Psychotoxic value of certain digestive disorders therapeutic trials. Ann Med Psychol (Paris). 1962 Dec; 120 (2): 721–726.
- Cazzola M, Tompkins TA & Matera MG. Immunomodulatory impact of a synbiotic in T(h)1 and T(h)2 models of infection. Ther Adv Respir Dis 2010; 4, 259–270.
- Collins Stephen M. , Michael Surette, Premysl Bercik. The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology 2012; 10, 735–742.
- Cryan John F. i Timothy G. Dinan. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, published online 12 September 2012.
- Demaude J, Salvador-Cartier C, Fioramonti J, Ferrier L, Bueno L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut 2006; 55, 655–661.
- Desbonnet Lieve, Lillian Garrett, Gerard Clarke, John Bienenstock ,Timothy G. Dinan. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of Psychiatric Research 2009; 43, 164–174.
- Desbonnet Lieve, Lillian Garrett, Gerard Clarke, B. Kiely, J.F. Cryan i Timothy G. Dinan. The effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression . Neuroscience 2010; 170, 1179–1188.
- Duerkop BA, Vaishnava S, Hooper L. V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity 2009; 31, 368–376.
- Forsythe P, Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol Invest 2010; 39, 429–448.
- Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock 2002; 18, 465–470.
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007; 56, 1522–1528.
- Girard Stéphanie Anne, Thierno Madjou Bah, Sévan Kaloustian, Laura Lada Moldovan, Isabelle Rondeau, Thomas A.Tompkins i wsp. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. British Journal of Nutrition 2009; 102, 10,1420–1425.
- Hemmings GP, Hemmings WA wyd. Biological Basis of Schizophrenia. Lancaster: MTP Press, 1978, 45–54.
- Hemmings GP, Hemmings WA wyd. Biological Basis of Schizophrenia. Lancaster: MTP Press, 1978, 37–44.
- Holdeman LV, Good IJ, Moore WE. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol 1976; 31: 359–375.
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 2007; 21: 374–383.
- Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet 2004; 20, 292–299.
- Javier A. Bravo, Paul Forsythe, Marianne V. Chew, Emily Escaravage, Hélène M. Savignaca, Timothy G. Dinan i wsp. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve PNAS 2011; 108, 38.
- Julianelle LA, Ebaugh FG, Implantation of Bacillus Acidophilus in persons with psychoses. Arch Neurol Psychiatr 1923, 9: 769–777.
- Kaloustian S, Wann BP, Bah TM, Falcao S, Dufort AM, Ryvlin P i wsp. Celecoxib after the onset of reperfusion reduces apoptosis in the amygdala. Apoptosis 2007; 12, 1945–1951.
- Kiliaan AJ, Saunders PR, Bijlsma PB, Berin MC, Taminiau JA, Groot JA i wsp. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. 1998 Nov; 275 (5 Pt 1): G1037–1044.
- Lizko NN. Stress and intestinal microflora. Nahrung 1987; 31: 443–447.
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 1995; 19: 11–38.
- Maes M, Kubera M, Leunis JC.The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008; 29 (1): 117–124.
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 702–721.
- Mayer, E. A. Gut feelings: the emerging biology of gut–brain communication. Nature Rev Neurosci 2011; 12, 453–466.
- Messaoudi Michael, Robert Lalonde, Nicolas Violle, Herve Javelot, Didier Desor, Amine Nejdi i wsp. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition 2011; 105, 755–764.
- Moore WE, Cato EP, Holdeman LV. Some current concepts in intestinal bacteriology. Am J Clin Nutr 1978; 31 (Suppl.): S33–42.
- Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. Journal of Affective Disorders 2005; 88 (2): 167–173.
- Myint AM, Kim YK, Vertek R. i wsp. Kynurenine pathway in major depression: evidence of impaired neuroprotection. Journal of Affective Disorders 2007; 98: 143–151.
- Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, i wsp. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 2006; 31, 1345–1355.
- O'Keane V, Dinan TG, Scott L, Corcoran C. Changes in hypothalamic–pituitary–adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol. Psychiatry 2005; 58 (12), 963–968.
- O'Toole PW i Cooney JC. Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip. Perspect Infect Dis 2008, art ID175285.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C i wsp. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464, 59–65.
- Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cellular and Molecular Life Sciences CMLSSeptember 2004, 61, 18, 2322–2331.
- Phillips JGP, The treatment of melancholia by the lactic acidbacillus. J Mental Sci 1910, 56: 422–431.
- Rao A Venket, Alison C Bested, Tracey M Beaulne, Martin A Katzman, Christina Iorio, John
- M Berardi i wsp. A randomized, double-blind, placebo controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathogens 2009, 1: 6.
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain gut–enteric microbiota axis. Nature Rev. Gastroenterol Hepatol 2009; 6, 306–314.
- Rudzki Leszek, Frank Monika, Szulc Agata, Gałęcka Mirosława, Szachta Patrycja, Barwinek Dominika. Od jelit do depresji – rola zaburzeń ciągłości bariery jelitowej i następcza aktywacja układu immunologicznego w zapalnej hipotezie depresji. Neuropsychiatria i Neuropsychologia 2012; 7, 2: 76–84.
- Singh MM, Kay S. Wheat gluten as a pathogenic factor in schizophrenia. Science 1976; 191: 191–192.
- Song C, Dinan T, Leonard BE. Changes in immunoglobulin, complement and acute chase protein levels in the depressed patients and normal controls. J Affect Disord 1994; 30: 283–288.
- Sudo Nobuyuki , Yoichi Chida, Yuji Aiba, Junko Sonoda, Naomi Oyama, Xiao-Nian Yu i wsp. Postnatal microbial colonization programs the HPA system for stress response in mice. J Physiol 2004; 558 (Pt 1): 263–275.
- Sudo N. Stress and gut microbiota: does postnatal microbial colonization program the hypothalamic–pituitary–adrenal system for stress response? Int Congr Ser 2006; 1287, 350–354.
- Suzuki K, Harasawa R, Yoshitake Y, Mitsuoka T. Effects of crowding and heat stress on intestinal flora, body weight gain, and feed efficiency of growing rats and chicks. Nippon Jujigaku Zasshi 1983; 45: 331–338.
- Thomas RH, Meeking MM, Mepham JR, Tichenoff L, Possmayer F, Liu S. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflamm 2012; 9, 153.
- Timoveyev I, Loseva E, Alekseeva T, Perminova N. Stability to sound stress and changeability in intestinal microflora. Eur Psychiatry 2002;17 (Suppl. 1): 200.
- Velin A , A-C Ericson, Y Braaf, C Wallon, J D Soderholm. Increased antigen and bacterial uptake in follicle associated epithelium induced by chronic psychological stress in rats. Gut 2004; 53: 494–500.
- Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC i wsp. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol 2007; 5, e156.
- Yang Ping-Chang, Jennifer Jury, Johan D. Soderholm, Philip M. Sherman, Derek M. McKay, Mary H. Perdue. Chronic Psychological Stress in Rats Induces Intestinal Sensitization to Luminal Antigens. American Journal of Pathology 2006; 168, 1.
- Wagar LE, Champagne CP, Buckley ND, Raymond Y, Green-Johnson JM. Immunomodulatory properties of fermented soy and dairy milks prepared with lactic acid bacteria. J Food Sci 2009; 74 (8).
- Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM i wsp. Ileal-lymphoid-nodular hyperplasia, non-specific colitis and pervasive developmental disorder in children. Lancet 1998; 351: 637–641.
- Wakefield AJ, Anthony A, Murch SH, Thomson M, Montgomery SM i wsp. Enterocolitis in children with developmental disorders. American Journal of Gastroenterology 2000; 95: 2285–2295.
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci 2012; 57, 2096–2102.
- Wann BP, Bah TM, Kaloustian S, Boucher M, Dufort AM, Le Marec N i wsp. Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: effects of sertraline. J Psychopharmacol 2009; 23, 451–459.
- Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-α-induced depression. J Psychiatry Neurosci 2004; 29: 11–17.
- Zareie M, K Johnson-Henry, J Jury, P-C Yang, B-Y Ngan, D M McKay i wsp. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006; 55: 1553–1560.
